In-house Products
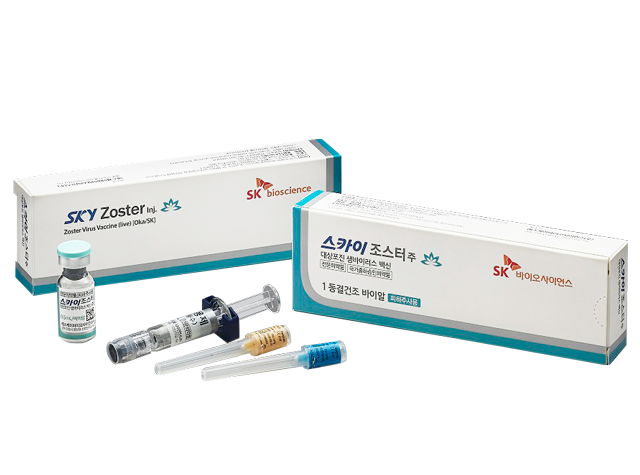
SKYZoster®
(Live Attenuated Vaccine)
- Classification Prescription medicine
- Therapeutics classificationZoster vaccine
- Ingredients/contentAttenuated varicella live virus
Description
Syringe prefilled with a colorless or pale-yellow liquid when reconstituted with lyophilized white crystalline pellet in a clear and colorless vial and an attached solvent (injection solution)
Dosage Forms and Strengths
Attenuated varicella live virus
Indications and Usage
- Prevention of herpes zoster in adults 50 years of age or older
Dosage and Administration
- This vaccine shall be injected subcutaneously at the lateral side of the upper arm as a one full dose (approximately 0.5 mL) and should not be injected intravascularly or intramuscularly.
- [Preparation and Administration Method]
- To dissolve this vaccine, add the entire amount of the attached solvent to the vial containing the lyophilized vaccine (using a 23G needle) and shake the vial until it is completely dissolved.
- Inject the entire amount (using 25 G needle) of this solution (approximately 0.5 mL) subcutaneously.
- To minimize loss of titer, the vaccine should be administered immediately after preparation, and any vaccine that has not been used within 30 minutes after preparation should be discarded.
How Supplied
1 lyophilized vial [attached solvent (prefilled syringe): 0.7 mL included] x in-house package unit 1 lyophilized vial [attached solvent (vial): 0.7 mL included] x in-house package unit (only applicable for export)
Storage
Keep refrigerated at 2-8°C in an airtight container away from light.
