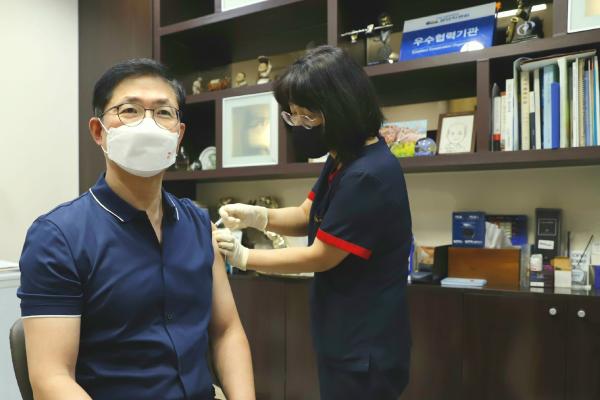
Global Innovative Partner of Vaccine and Biotech SK bioscience
From Prevention To CureOur Science & Technology
Conducting research into vaccines that will be the driving force in the life science business going forward.
The world's best vaccine factory with advanced vaccine technology.
Leading in expanding the global market through cooperation with global companies.
Investor Relations
0
PRESENT PRICE (WON)
0WON
0.00%
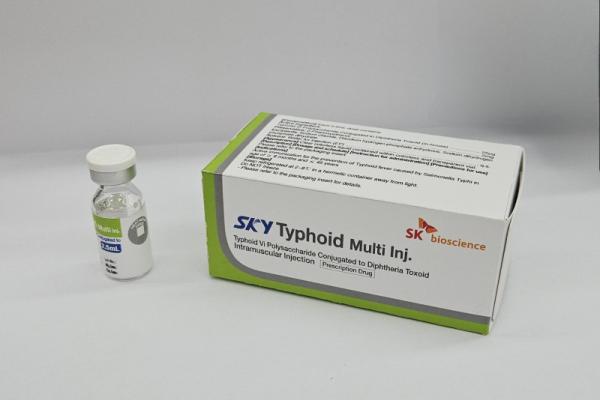
Our Products
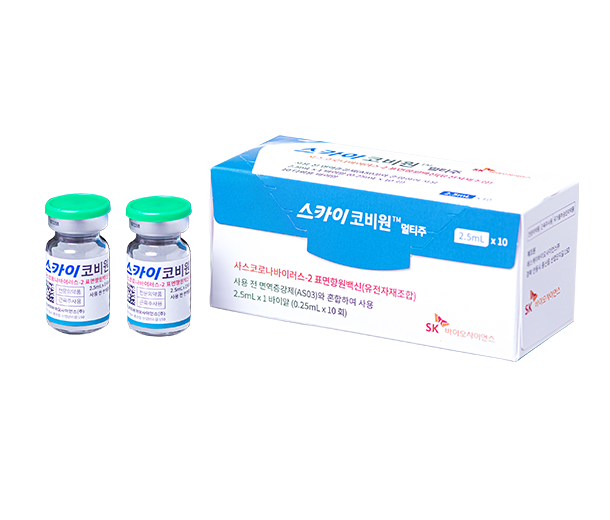
- SKYCovione™
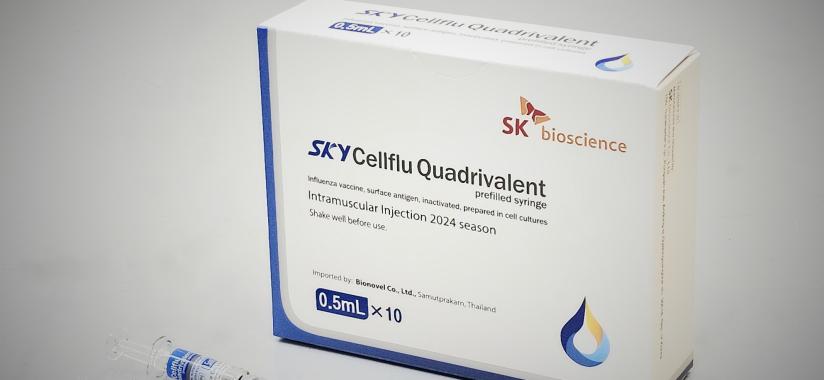
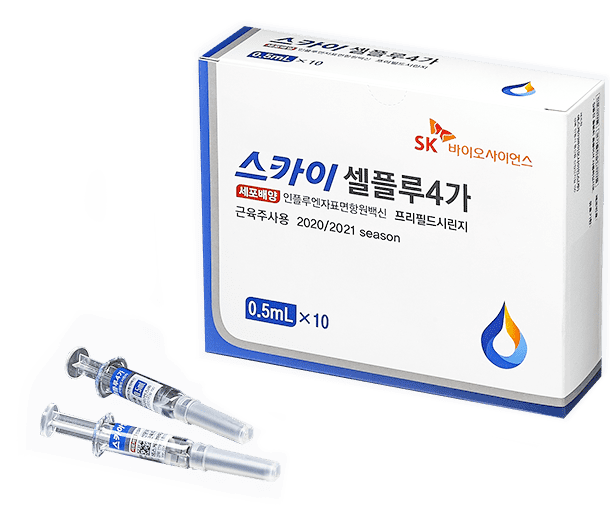
- SKYCellflu® Quadrivalent Prefilled Syringe
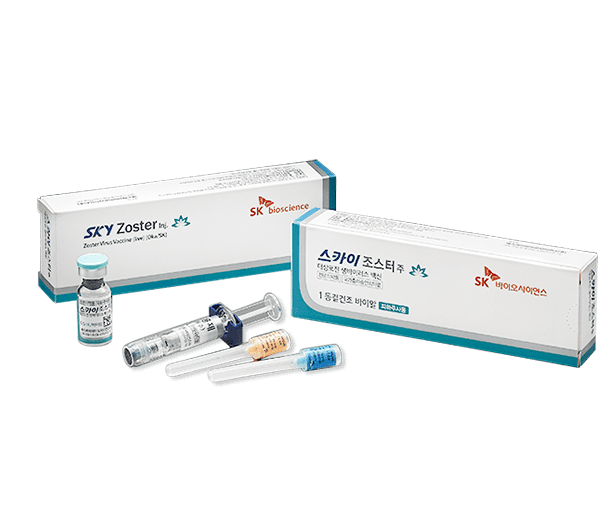
- SKYZoster®
- SKYVaricella®